Soluble starch powder is obtained from native insoluble starch which originates from Solanum tuberosum.
CAS Number : 9005-84-9
Molecular Weight : 342.30
Molecular Formula : C12H22O11
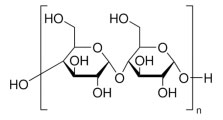
Soluble starch is a colorless powder that finds widespread application across various research domains owing to its polysaccharide nature.
Soluble Starch
Soluble starch acts as a thickener in food products. When added to soups, sauces, and gravies, it enhances their consistency and texture. It helps maintain uniformity and stability, preventing gel breakdown during processing.
Modified soluble starches are used as water-soluble gelling agents. They stabilize high-shear emulsions, such as mayonnaise and salad dressings, ensuring their desired texture and shelf life.
Hydrolyzed starch (such as maltodextrin) serves as a fat replacer in low-fat spreads, mayonnaise, and ice cream. It provides texture and mouthfeel similar to fats while reducing overall fat content.
Soluble starch contributes to the overall quality of baked goods, confectioneries, and pastas. It improves texture, shelf life, and sensory attributes.
In summary, soluble starch plays a vital role in achieving desirable properties in various food products, from thickening soups to enhancing the stability of emulsions.
In enzymology studies, it acts as a substrate for enzymes like amylase, enabling research of enzymatic behavior & kinetics. This usage is pivotal for comprehending the breakdown & transformation of starches within biological systems. In microbiology, soluble starch is incorporated into culture media to explore the metabolic pathways of microorganisms reliant on starch as a carbon source.
In materials science, its adhesive & film-forming characteristics are exploited to engineer biodegradable materials & coatings.
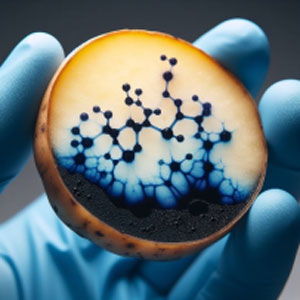
In analytical chemistry, starch is used as qualitative indicator of iodine & vice versa. This is due to the fact that starch can form complexes with it which are of a distinctive blue-black color.
Latent Fingerprints :
Latent fingerprints are invisible prints left behind when a person touches a surface. These prints consist of natural oils, sweat, and other substances from the skin. Detecting latent fingerprints is essential for solving crimes, identifying suspects, and linking individuals to specific locations or objects.
Starch-Iodine Reaction :
The reaction involves the interaction between iodine vapor and the fatty acids present in latent fingerprints.
Here’s how it works :
Visualization Process :
Investigators use a starch solution (usually containing iodine) to visualize latent fingerprints. The starch solution is applied to the surface where the fingerprint is suspected to be present. If a latent fingerprint is there, the iodine in the starch solution reacts with the fatty acids, forming a brown color. The brown color highlights the ridges and patterns of the fingerprint, making it visible.
Besides latent fingerprints, the starch-iodine reaction is also used to detect lipid-containing substances (such as oils or greases) at crime scenes. In summary, the starch-iodine reaction provides a valuable tool for forensic investigators to reveal hidden fingerprints and aid in criminal investigations.
Appearance : Colorless to off-white powder
pH of 2% solution at 25°C : 5.0 to 7.0
Residue on Ignition (Ash) : ≤ 0.4%
Solubility : Complies using the below method:
Prepare a paste of 2.0 g of the sample with a little cold water & add to it with stirring 100 mL of boiling water. The solution should be no more than opalescent, and on cooling it should remain liquid and not increase in opalescence.
Sensitivity : Complies using the below method:
Starch solution : Triturate 1.0 g of soluble starch with 5 ml of water & whilst stirring pour the mixture into100 ml of boiling water containing 10 mg of mercuric iodide.
Iodine solution : To 10 ml of 0.0 M iodine solution, add 0.6 g of potassium iodide & dilute to 100 ml with water. Prepare immediately before use.
Test for sensitivity : To a mixture of 1 ml of above Starch solution and 20 ml of water, add about 50 mg of potassium iodide & 0.05 ml of iodine solution. The solution is blue.