Octenyl succinate starch sodium (OSS), INS1450, is a modified starch, used as an excipient to provide benefit formulations of food, nutraceutical & pharmaceutical products. OSS is derived as a white or nearly white powder or granules, or, if pre-gelatinized, as flakes, amorphous powder, or coarse particles.
OSS is derived from native starch (such as corn, potato, or tapioca) through esterification with octenyl succinic anhydride (OSA) in presence of alkali as shown below
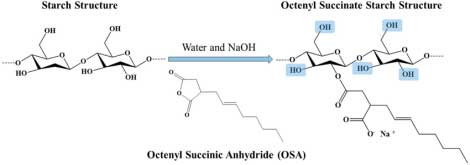

Octenyl Succinate Starch
OSS is an emulsifier, stabilizer, solubilizer & thickening agent. The modification enhances product stability, texture & quality functional properties as can be seen in the following table :
| Oral Formulations | Functions | Properties of OSS |
|---|---|---|
|
|
|
OSS is recognized as safe (GRAS) by regulatory agencies such as the USFDA. Modified starch, INS 1450, is well tolerated in humans up to a single dose of 25,000 mg/person. However, manufacturers must adhere to specific usage levels and labelling requirements.
Coffee, Coffee Substitutes, Tea, Herbal Infusions, & Other Hot Cereal & Grain Beverages (excluding Cocoa)
This includes ready-to-drink products (e.g., canned), as well as their mixes and concentrates. Examples consist of chicory-based hot beverages (like a powdered roasted grain beverage popular as a coffee substitute), rice tea, mate tea, and blends for hot coffee and tea beverages (e.g., instant coffee, powder for hot cappuccino drinks). Treated coffee beans for coffee product manufacturing are also encompassed. Ready-to-drink cocoa and cocoa mixes are incorporated.
Maximum Level : Good Manufacturing Practices (GMP)
Notes : OSS is intended for use in ready-to-drink products & pre-mixes specifically designed for ready-to-drink products.
Complementary Foods for Infants and Young Children
These foods are designed for infants aged 6 months and older, as well as for gradually transitioning infants and children to regular foods. Products may be in a ready-to-eat form or as powder to be reconstituted with water, milk, or another appropriate liquid. These foods do not encompass infant formulae, follow-up formulae, or formulae intended for special medical purposes.
Examples include cereal-based, fruit-based, vegetable-based, and meat-based "baby foods" for infants, "toddler foods," and "junior foods," as well as lacteal flour, biscuits, and rusks for children.
Maximum Level : 50,000 mg/kg
Note on usage :
Fermented Milks (Plain), Heat-Treated After Fermentation
This category includes both fluid and non-fluid plain products, such as yoghurt and plain drinks based on fermented milk. These products have undergone heat treatment (e.g., sterilization or pasteurization) after the fermentation process.
Maximum Level : Good Manufacturing Practices (GMP)
Note : Permissible for use exclusively as a stabilizer or thickener.
Fermented Milks (Plain), Not Heat-Treated After Fermentation
This category comprises fluid and non-fluid plain products, including yoghurt and plain drinks based on fermented milk that have not undergone heat treatment after fermentation.
Maximum Level : Good Manufacturing Practices (GMP)
Note : For use as a stabilizer or thickener only, and exclusively in reconstituted and recombined products.
Formulae for Special Medical Purposes for Infants
These foods are intended for special dietary use and are specially processed or formulated for the dietary management of infants. They should only be used under medical supervision. These formulae are designed for the exclusive or partial feeding of infants who have limited or impaired capacity to consume, digest, absorb, or metabolize regular infant formulae or specific nutrients contained therein, or those with other medically determined special nutrient requirements. The dietary management cannot be achieved solely by modifying the normal diet, using other foods designed for special dietary purposes, or a combination of both.
Maximum Level : 20,000 mg/kg
Note :
Frozen Egg Products
This pertains to purified whole eggs, egg yolks, or egg whites that are pasteurized and subsequently frozen.
Maximum Level : Good Manufacturing Practices (GMP)
Liquid Egg Products
This encompasses purified whole eggs, egg yolks, or egg whites that are pasteurized and chemically preserved, such as through the addition of salt.
Maximum Level : Good Manufacturing Practices (GMP)
Pasteurized Cream (Plain)
This category comprises cream that has undergone pasteurization through appropriate heat treatment or is produced from pasteurized milk. It includes milk cream and "half-and-half."
Maximum Level : Good Manufacturing Practices (GMP)
Note : Excludes the products conforming to the Standard for Cream and Prepared Creams, including reconstituted cream, recombined cream, and pre-packaged liquid cream (CODEX STAN 288-1976).
Renneted Milk (Plain)
This category includes plain coagulated milk produced through the action of milk coagulating enzymes. It covers curdled milk, and flavored renneted milk products are found in dairy-based desserts (e.g., pudding, fruit, or flavored yoghurt).
Maximum Level : Good Manufacturing Practices (GMP)
Sterilized and UHT Creams, Whipping and Whipped Creams, and Reduced-Fat Creams (Plain)
This category incorporates all types of cream, irrespective of their fat content, which have undergone more extensive heat treatment than pasteurization. It includes pasteurized creams with reduced fat content and creams intended for whipping. Sterilized cream undergoes proper heat treatment within the container presented to consumers. Ultra-heat treated (UHT) or ultra pasteurized cream undergoes the appropriate heat treatment (UHT or ultra pasteurization) in a continuous flow process and is aseptically packaged. Cream may also be packaged under pressure (whipped cream). This category encompasses whipping cream, heavy cream, whipped pasteurized cream, and whipped cream-type dairy toppings and fillings. Creams or toppings with partial or complete replacement of milk fat by other fats are also included.
Maximum Level of usage : Good Manufacturing Practices (GMP)
The Codex General Standard for Food Additives (GSFA) defines the conditions for the utilization of Starch sodium octenyl succinate in food products. These provisions apply within the framework of good manufacturing practices (GMP), as described in the introduction of the Codex GSFA.
Note that although not explicitly mentioned, OSS can also be used in heat-treated buttermilk (under Fluid milk (plain)) and in spices (under Herbs and spices).
OSS sodium (INS1450) can be used in a wide range of food categories, including but not limited to :